Corrosion Control
Corrosion Overview
Corrosion involves the loss of metal, usually iron, to its environment. If conditions are nonoxidizing, this dissolved iron may remain in solution in the form of ferrous iron, imparting a green tint to the CBF. However, if conditions are oxidizing, the dissolved iron may further oxidize to the ferric ion, which will commonly form reddish brown ferric hydroxide and cause formation damage.
In the corrosion process, the metal loses electrons and some other species—such as an oxygen molecule or a hydrogen ion—accepts them. The site where the loss of electrons takes place is called the anode. The site where the electrons are accepted is called the cathode. These sites are often at different locations on the metal with the electrons being shunted through the metal as shown in Figure 20.
For electron migration to occur, two other aspects are needed: (1) a conductive phase, in this case the metal and (2) an electrolyte, which acts as a medium for the electrolytic reactions and facilitates the removal of the corrosion products from the corroding surface.
For corrosion to take place, these four components of the corrosion system must coexist:
- an anode—a susceptible area on the metal surface,
- a cathode—oxygen or other electron acceptor,
- a conductive path—the body of the tubing or casing, and
- an electrolyte—field brine or CBF.
FIGURE 20. The Corrosion Process
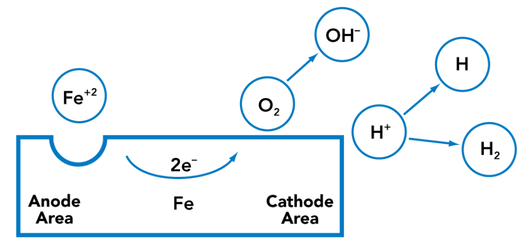
An important facet of inhibiting or minimizing corrosion is controlling the electrode processes—the phenomena occurring at the anode and cathode. If these processes can be interrupted in some manner, the corrosion rate will be dramatically reduced. Since the anode and cathode reactions depend on one another, any means which hinders either reaction will also hinder the corrosion. Removing or reducing the effective concentrations of one or more of the reactants will accomplish this.
One can decrease corrosion by covering the surface of the metal with a physical barrier such as a chemical coating or by reducing the reactivity of the iron by what is called passivation.
Inhibiting the cathodic reaction is accomplished by reducing the concentrations of corrosion accelerators such as oxygen molecules, hydrogen ions, and other metallic ions.
The cathodic reaction can be interrupted by:
- keeping oxygen out of the system,
- reducing the concentration of hydrogen ions by increasing the system pH, and
- removing accelerators like ferric ions.
Oxygen Control
The addition of one of TETRA's oxygen scavengers, like OxBan or OxBan HB, can reduce corrosion dramatically by limiting the reaction of oxygen at the cathode—less oxygen, less reaction. With respect to corrosion control, oxygen can be entrained in brine fluids and pumped as small bubbles (or foam) during recirculation and filtration procedures. Therefore, generation of foam during these operations should be minimized. Additionally, small amounts of an oxygen scavenger can be added continuously just before the fluid goes downhole. More effective corrosion inhibition is achieved by employing a combination of one of the oxygen scavengers and a corrosion inhibitor like TETRAHib or TETRAHib Plus.
If the fluid is not under pressure, oxygen solubility in brines is reduced with an increase in temperature. However, with applied pressure, oxygen cannot escape from the fluid as the temperature rises.
Oxygen control is extremely important in minimizing environmentally assisted cracking. Consequently, the scavenger and treatment level must be chosen carefully. For more information, see "Environmentally Assisted Cracking."
|
OxBan should not be used with calcium or zinc containing brine fluids and should be avoided with CRAs. OxBan HB is an excellent alternative that avoids potential precipitation and acidity issues. |
Hydrogen Ion Reduction
The hydrogen ion is a corrosion accelerator, and the concentration of hydrogen ions can be controlled during the manufacturing process. All TETRA clear brine fluids are carefully monitored to control pH during manufacturing.
Other Contributors to a Corrosive Environment
- High Pressure/High Temperature
- Scale (e.g., CaCO3, CaSO4, FeS, ZnS)
- Sulfur Producing Species/Bacteria (e.g., H2S sources)
- Metallurgy
- Stress
- Mechanical Deformations/Crevices
Major Types of Corrosion
Corrosion is a complex subject involving a broad spectrum of materials, chemistries, and practices. A brief discussion of some of the more pertinent types of corrosion follows. Two major types of corrosion are general (or uniform) corrosion and localized corrosion. These are discussed in the following sections.
General Corrosion
Uniform or general corrosion is corrosion that is uniformly or widely distributed across the surface of the metal. With the loss of metal occurring across a large (if not the entire) surface of the metal, uniform corrosion rates are much higher than local corrosion rates. Perhaps the most serious general corrosion type, since it is often not anticipated, is galvanic corrosion, which occurs due to the interfacing of two different metals. The potential or galvanic difference between the two metals—the ease with which they give up electrons—induces a cathodic/anodic reaction between the two metals.
Localized Corrosion
Localized corrosion is corrosion that is confined to small, specific sites on the surface of the metal. This type, although limited to a small region, can be especially insidious. Localized corrosion can proceed inward from the surface of the metal. In extreme cases, it can ultimately lead to cracking or failure of the metal. Localized corrosion, which leads to small dot-like regions or cavities on the surface of the metal, is called pitting. Pitting may have serious consequences, since corrosion may easily continue deeper into the metal, owing to the development of a concentration cell.
More specific types of localized corrosion are described in detail below, including:
- Concentration Cell Corrosion (crevice, etc.)
- Hydrogen Embrittlement (hydrogen induced cracking
- Environmentally Assisted Cracking
Concentration Cell Corrosion
Concentration cell corrosion results from differences in electrical potential that develop due to differences in the concentration of dissolved ions in CBFs. This is perhaps the most common type of corrosion because, invariably, small concentration differences exist in different locations throughout the fluid due to factors like temperature variations and mixing limitations. Crevice corrosion is a specific type of concentration cell corrosion that results from different concentrations of species inside and outside the crevice. The key species are often oxygen or hydrogen, but they can also be nonreactive ions. Corrosion due to concentration differences can be severe in the bottom of a pit or cavity.
Hydrogen Embrittlement—Hydrogen Induced Cracking
Hydrogen induced cracking (HIC) results from the migration of hydrogen atoms from the metal surface into the metal lattice, followed by the recombination of hydrogen atoms to form hydrogen gas. The gas either causes blistering on the metal surface or induces cracking along weak matrix elements, such as inclusions or grain boundaries. The probability of induced cracking can be enhanced by stress, leading to a stacking of cracks, transgranular cracking, and possible tubing failure. Reduced acidity and reduced sulfur content decreases the metal's susceptibility to hydrogen induced cracking.
Environmentally Assisted Cracking
The term, environmentally assisted cracking (EAC), encompasses all of the forms of stress corrosion, whether such corrosion is induced by hydrogen embrittlement, chlorides, other halides, or sulfides. To initiate EAC, three essential conditions must be present: (1) stress in the tubing, (2) a sensitive metallurgy, and (3) a corrosive environment. Minimizing the potential for catastrophic failure in today's HPHT wells is a difficult challenge, because all three conditions necessary to initiate EAC are often present in such wells.
It should be noted that the tensile stress needed to induce cracking and potential tubing failure can be substantially below the metal's indicated tensile strength. Moreover, the stresses that may contribute to a cracking event can come from either the external forces acting on the pipe, such as the mechanical stresses generated as a result of its service in the well, or from internal stresses residual from any of the metal's manufacturing process, such as cold working, drawing, rolling, or annealing. Cracking is not normally accompanied by general corrosion of the tubing's surfaces; instead, it often emanates from deep invasive pitting. In some cases, the propagation of cracking can be extremely rapid with catastrophic failure occurring in as little as a few days. However, catastrophic failure can also take months or even years to occur.
The use of corrosion resistant alloys has markedly increased with the growth in drilling HPHT wells. Where general corrosion was the concern with carbon steel tubing, environmentally assisted cracking has become the blight of the stainless steels. The austenitic 300-series stainless alloys such as 304 SS and 316L SS are particularly susceptible to EAC. Even the normally greater corrosion resistant martensitic 13 chrome and duplex (ferritic/martensitic) chrome alloys have experienced failure when stressed in company with certain corrosive fluids. The threat of EAC has become a significant problem since most of the CRA materials in use today are martensitic 13 chrome alloys of various composition.
Many of the tubing failures in the last five to 10 years have been attributed to stress cracking or EAC that has been induced by the corrosive environment in the annulus (from the back or packer fluid side). This type of cracking can more precisely be termed annular environmentally assisted cracking (AEAC) to distinguish it from the EAC that is induced by the production fluids on the inside of the tubing.
EAC or AEAC events are usually categorized as being either of two types: (1) stress corrosion cracking (SCC) or (2) sulfide stress cracking (SSC). By examining crack patterns and debris deposited in cracks, metallurgists can often identify the cracking type. In some cases, however, the cracking mechanism appears to involve a blend of the two. Overviews of these two types are provided in the following section:
Stress Corrosion Cracking. Stress corrosion cracking (SCC), often referred to as halide or chloride stress cracking, depends upon a variety of factors such as the metallurgy; the pH; concentration levels of CO2, oxygen, and sulfur; the temperature; the halide (chloride or bromide) content; and applied or residual stresses. As a general rule, the susceptibility for this type of cracking increases with higher oxygen content, increased halide concentration, and elevated temperature. In view of the complexity of the interaction of these factors, TETRA does not specify general halide concentration maximums, but takes a total system approach to minimizing this type of corrosion.
With respect to pH, the presence of high concentrations of chloride or bromide ions can locally enhance the acid concentration by reaction of the halide ions with metal ions in crevices or under scale. Such concentration effects can then initiate concentration cell corrosion and pitting—the latter often being the genesis of cracking. For additional discussion of the effect of the halide ion, see "Salinity Concentration" in the "Key Corrosion Factors" section.
Sulfide Stress Cracking. Sulfide stress cracking (SSC) results from the presence of hydrogen sulfide (H2S) in the fluid environment. This toxic gas elevates the acidity of the fluid, increasing its corrosivity. It also supplies a sulfide ion that can either be readily oxidized to elemental sulfur or can react with various heavy metals to precipitate metal sulfide scale. The insolubility of the sulfides and elemental sulfur can lead to localized corrosion or concentration cell corrosion with high acid concentrations being trapped under the solid deposits. Sulfur is invariably found in association with high concentrations of hydrogen sulfide. Moreover, hydrogen sulfide has been found to react with elemental sulfur to form a particularly corrosive species.
SSC is a specialized form of hydrogen induced cracking or embrittlement. The presence of H2S promotes the movement of hydrogen atoms from the metal's surface into the metal's matrix. This induces the hydrogen atoms to form hydrogen gas (H2) at points of stress in the metal, for instance, at the grain boundary or at an inclusion in the metal's matrix, which can then lead to cracking. The hydrogen embrittlement component is more prominent in the carbon and low alloy steels than in the martensitic CRA steels. As a general rule with low alloy steels, reducing the acidity will inhibit SSC. High temperatures will also lower the chance for SSC. Maximum susceptibility for SSC in martensitic stainless steels occurs at approximately 68°F.
Fluid/Metal Compatibility Testing—MatchWell Fluid Compatibility Selector
Because the use of CRA tubular materials is a relatively new practice, there is little empirical data to draw upon when making completion decisions. Most of the conventional wisdom on the matter comes from the extrapolation of data from conventional completions, miscellaneous observations of failures, and a minimum of test data. In an effort to better serve our customers, TETRA has participated in extensive testing to learn more about AEAC and how to reduce its probability.
In this research effort, TETRA has conducted comprehensive matrix testing under a variety of downhole conditions: e.g., select chrome tubing under high stresses, temperatures up to 400°F, typical completion and packer fluids across the full density range from 9.0 lb/gal to 19.2 lb/gal. The testing also examined the impact contamination from corrosive gases and other materials would have. Using the test findings, TETRA created the MatchWell fluid compatibility selector, which can be used to match any tested CRA tubing metallurgies with TETRA CBFs. This computer program provides cracking susceptibilities indices (CSIs) for various metallurgy and fluid combinations. The MatchWell program allows TETRA to provide operators with the information and opportunity to select tubing and fluids that have been tested and matched for optimal performance and cost effectiveness.
Key Corrosion Factors
With respect to AEAC, careful attention must also be given to several properties of the CBFs. Four factors are of primary importance: (1) the salinity concentration, (2) the oxygen content, (3) the pH, and (4) the presence of CO{}2, H2S, or sulfide levels.
Salinity Concentration. The salinity, or halide concentration, is significant to the potential for AEAC, particularly stress corrosion cracking, in that it is implicated in the formation of localized corrosion (pitting). As this corrosive process proceeds, due to the presence of halides, very low pH can be developed within the pit, leading to more severe pitting and possibly to cracking of the metal. Although there have been attempts to assign definitively safe halide concentrations for corrosion resistance, this practice is best avoided, since TETRA's test findings point to the importance of other minor variables such as contaminants, metallurgical variations, and other unexpected factors as contributive to SCC.
Chlorides, and not bromides, are frequently implicated in SCC events. However, our testing indicates that, in certain conditions, bromide fluids can exhibit SCC, while fluids containing chlorides can perform exceptionally well.
For a given metallurgy and stress level, the halide ion concentration that becomes pernicious with respect to stress cracking depends on temperature and the concentration of CO2 and/or H2S. At temperatures of 200°F and greater, the potential for cracking becomes highly dependent on the halide concentration. Regardless of the halide concentration, increased hydrogen sulfide and/or CO2{~} intensifies the potential for cracking.
Oxygen Content. There is no indicated minimum limit for oxygen content that will ensure that there is no risk of AEAC. Cracking has been reported in fluids with less than 0.5 ppm oxygen; however, our extensive testing suggests the detrimental influence of other substances in these failures. This conclusion stems from the fact that, in a number of our tests, high levels of oxygen were present but no failures occurred. In general, many studies have concluded that minimizing oxygen content in the fluid is advisable. The combination of oxygen with H2S, or more significantly the S-2 (or HS-1) ion found in neutral to alkaline H2S solutions, is especially dangerous with respect to AEAC.
Due to the potential for pitting and severe localized corrosion, strict oxygen control by the application of oxygen scavengers should be viewed as imperative to AEAC inhibition. As a general rule, it is always advisable to ensure that oxygen scavenger levels are optimized during final circulation prior to setting the packer.
pH. At low pH (pH <7), the hydrogen ion (H+) can be converted at cathodic sites to atomic or molecular hydrogen and, coupled with stress, can lead to HIC. Additionally, at low pH, highly localized, strongly acidic sites can develop and lead to pitting. As a consequence, the pH of the brines should be kept alkaline (pH >7), if possible, to prevent these occurrences.
CO2, H2S, or Sulfide Level. It is important to minimize the H2S or sulfide level to avoid the formation of sulfur and related species at pitted sites. The use of sulfide scavengers or biocides to inhibit sulfate reduction can be enormously helpful. Similarly, an increase in CO2 concentration also heightens the potential for cracking. The most likely influence of the CO2 is its propensity to lower the pH of the fluid environment.
Metallurgical Issues
Acting on generalizations about metallurgies that will resist AEAC can be dangerous. Our testing program has identified a number of combinations of chrome tubing and CBFs that are compatible in the harshest of downhole conditions. Higher chrome or other exotic alloyed materials are often selected for use with the hope that these will be impervious to the assault of halide ions and other corrosive substances. Testing, however, has shown that this practice does not always result in the best technical solution; it also can be very expensive.
Even at high temperatures and in the presence of known corrosion enhancers like H2S and oxygen, lower order CRAs and chloride fluids can perform as well as exotic alloys and bromide fluids. In many cases, small concentrations of contaminants, formerly considered insignificant, contribute to AEAC. Often, it is a combination of two or more factors working synergistically to the metal's demise. TETRA's comprehensive matrix testing has identified many significant factors that can provide guidance when making completion decisions and selecting materials.
