MicroBio MB1 Bioaerosol Sampler - Operating Manual
For products used before October 2023 please contact Cantium Scientific Ltd or refer to the instructions supplied with the device.
- 1. Regulatory Compliance
- 2. WEEE and Recycling
- 3. Warranty
- 4. MicroBio MB1 Technical Specification
- 5. Introduction
- 6. Battery and External Power
- 7. IQ / OQ / PQ
- 8. Sampling
- 9. Determining Results
- 10. Change Plate / Dish Type
- 11. Cleaning
- 12. Validation
- 13. Calibration
- 14. Troubleshooting
- 15. Appendix A - 220 Hole Count Correction Table
- 16. Appendix B - 400 Hole Count Correction Table
- 17. Appendix C - Culture Media Types
- 18. Appendix D - Replacement Parts
1. Regulatory Compliance
EC Declaration of Conformity
This is to certify that MicroBio MB1 products manufactured from April 2017 comply with the essential requirements of the following European Community Directives when used according to their intended purpose:
Electromagnetic Compatibility (EMC) Directive 2014/30/EU
BS EN 61326-1:2013 - Scientific, Test and Measurement Equipment
Referencing:
BS EN 55011/CISPR11 - Emissions for Industrial, Scientific and Medical Equipment
BS EN 61000-4-2 - Immunity to Electrostatic Discharge
BS EN 61000-4-3 - Radiated Immunity
Restriction of the Use of Certain Hazardous Substances (RoHS-3) in Electrical and Electronic Equipment (EEE) Directive (EU 2015/863).
2. WEEE and Recycling
At the end of this product's life, please dispose at an appropriate recycling centre, or return it to your local distributor who, depending on local laws, may be obliged to take the product for safe recycling and disposal.
The MicroBio MB1 key parts are manufactured from the following:
Part |
Material |
Recyclable |
Blower housing |
Aluminium |
Yes |
Air blower * |
Body and rotary vanes: Glass reinforced nylon
Motor: Steel, copper, rare earth magnets, and others |
Yes (limited facilities) |
Enclosure |
ABS Plastic |
Yes |
Display window |
Polycarbonate |
Yes |
Tripod mount |
Aluminium |
Yes |
Screws and washers |
Stainless steel |
Yes |
Circuit board, wires * |
Various |
Partially |
Sampling heads |
Stainless steel or aluminium |
Yes |
Carry case |
ABS Plastic |
Yes |
Packaging |
Cardboard and PET |
Yes |
Documentation |
Paper |
Yes |
Batteries * |
NiMH |
Partially |
* These items must be taken to approved recycling centres equipped to handle such waste and recover the metals used in their manufacture. Some elements of these parts cannot be recycled.
3. Warranty
The manufacturer warrants this product to be free from defects in materials and workmanship for 36 months from the date of purchase.
If your product is found to be defective within that period, please contact Cantium Scientific Limited or your local distributor who will arrange for repair of the instrument, or if necessary a replacement.
This warranty does not cover accidental damage, wear and tear, incorrect use, consequential or incidental loss. The warranty excludes rechargeable cells supplied with the sampler.
Damage caused by cleaning materials and methods not recommended by the manufacturer, use beyond the specification, use in wash down areas (unless used in approved protective bags), or modifications without prior permission from the manufacturer will invalidate the warranty.
This warranty does not affect your statutory rights.
4. MicroBio MB1 Technical Specification
Parameter |
Standard Model |
HiFlow Model |
Flow Rate ‡ |
100 L.min-1 |
180 L.min-1 |
Sample Volume |
10 to 2,000 litres in varying steps |
|
Sampling Volume Capacity * |
~ 60,000 litres before recharge |
~ 40,000 litres before recharge |
d50 Particle size |
1.7 µm (220 x 1mm head) 1.35 µm (400 x 0.7mm head) |
1.7 µm |
Mean particle velocity |
9.62 ms-1 (220 x 1mm head) 10.7 ms-1 (400 x 0.7mm head) |
9.55 ms-1 |
Other Features |
Auto switch off Delayed start up to 99 minutes 4-digit 7-seg LED display Sample cancel feature Padded carry case supplied |
|
Dimensions |
197 x 105 x 94.5 mm (inc. head) sold to August 2025 197 x 105 x 101mm (inc. head) sold from September 2025 |
|
Power |
4 x AA NiMh Cells |
4 x AA NiMh Cells |
Noise Level |
< 75dB @ 1m |
< 80dB @ 1m |
Environmental Operating Range |
-10 to 50°C up to 90% RH‡ |
|
Sampling Plate |
55mm/65mm contact plate or 90mm petri dish |
90mm petri dish |
Sampling Head |
316 grade stainless steel 220 x 1mm holes or Anodised aluminium 400 x 0.7mm holes or 316 grade stainless steel 400 x 0.7mm holes |
316 grade stainless steel 400 x 1.0mm holes |
* Based upon random samples until low battery warning given. These tests were undertaken on units fitted with new and fully charged Ansmann Max-e 2500 mA.Hr NiMh cells. Actual battery life may vary due to volume taken per sample, interval between samples, age of cells, and other environmental effects, such as humidity and temperature.
‡ Calibrated at 1013mbar 20ºC. Environmental conditions will affect air pressure thus mass/volumetric flow rates.
5. Introduction
The MicroBio MB1 is part of the MicroBio range of bioaerosol samplers and is one of the most economical portable samplers in the world for monitoring airborne micro-organisms or bioaerosols.
The MicroBio range meets the standard required for a reference sampler, as fully validated by the UK Department of Trade and Industry Validation of Analytical Methods (VAM) programme. They are designed and tested to meet the requirements of ISO14698, EN 17141-2020 and USP797 requirements.
The sampler collects airborne micro-organisms by drawing a stream of air at a constant flow rate through a series of small holes in a metal head. Particles suspended in the air stream impinge onto the surface of a sterile culture medium in a contact plate or petri dish.
After exposure to a set volume of air, the contact plate or petri dish is removed and incubated. The number of colonies which develop are counted, enabling a calculation to be made to determine the concentration of micro-organisms in the air (CFU / m3 - colony forming units per cubic metre).
6. Battery and External Power
6.1. Installing Battery
The battery is held in a compartment at the back of the MicroBio MB1. The unit is supplied with 4 x AA NiMH rechargeable cells. The cells must be removed for re-charging.
To open the battery compartment press firmly on the “OPEN” marking on the battery compartment lid near the serial number label and then slide downwards.

The orientation of the cells is indicated within the battery compartment.
Carefully replace the lid with an upwards sliding motion ensuring the “OPEN” marking on the battery compartment lid is towards the serial number label.
NOTE:
Please read the instructions supplied with the charger before charging the NiMh cells. To avoid corrosion, we recommend cells are removed from the unit if it is to be left unused for extended periods and fully charged before use.
6.2. External Power (optional)
Available as an optional extra, the MicroBio MB1 can be powered from an external 5V to 9V DC source. When this option is fitted, a USB type A to 2.1 mm DC power jack cable is supplied that enables use of the battery charger power supply as the external power source. The cable also enables the MicroBio MB1 to be powered for any other USB type A power source rated at 2.4A.
The DC power inlet connector on the MicroBio MB1 is located as shown below:

The external power will not charge the battery inside the MicroBio MB1. However, both power sources can be used simultaneously as protection circuitry inside the MicroBio MB1 will prevent one source from feeding into the other.
6.3. Low Battery Indication
When the battery needs re-charging the display will show bat lo on the display. If the battery level is too low, the MicroBio MB1 will not allow samples to be taken, display bat flat on the display and switch off.
Holding down the stop button will get the display to show a battery level percentage.
7. IQ / OQ / PQ
Documentation templates are available from Cantium Scientific Limited or your local distributor to support in-house Installation, Operational and Performance Qualification.
Customised templates are available upon request.
8. Sampling
The selection of the sample volume is important for reliable sampling. If the contact plate or petri dish is overloaded with colonies it is difficult to make an accurate count. With experience, the user will anticipate the probable bioaerosol concentration in an area, but it may be necessary to make a preliminary survey at a number of sampling volumes to identify the optimum setting. Each sample should be repeated several times and a statistical mean value and confidence value determined.
8.1. Selection of Media
The agar media used in the contact plate or petri dish should be chosen to suit the micro-organisms being monitored. For a wide range of micro-organisms, consider using tryptone soy agar (TSA), casein soy peptone agar (CPSA) or nutrient agar (NA). There are other selective agars for more specific micro-organisms. For fungi (yeasts and moulds), consider using malt extract agar (MEA) or rose bengal agar (RBA). Appendix C details various culture media types.
8.2. Inserting a Contact Plate or Petri Dish
ALWAYS STERILISE THE SAMPLING HEAD PRIOR TO EACH USE.
USE OF HYGIENE GLOVES IS RECOMMENDED
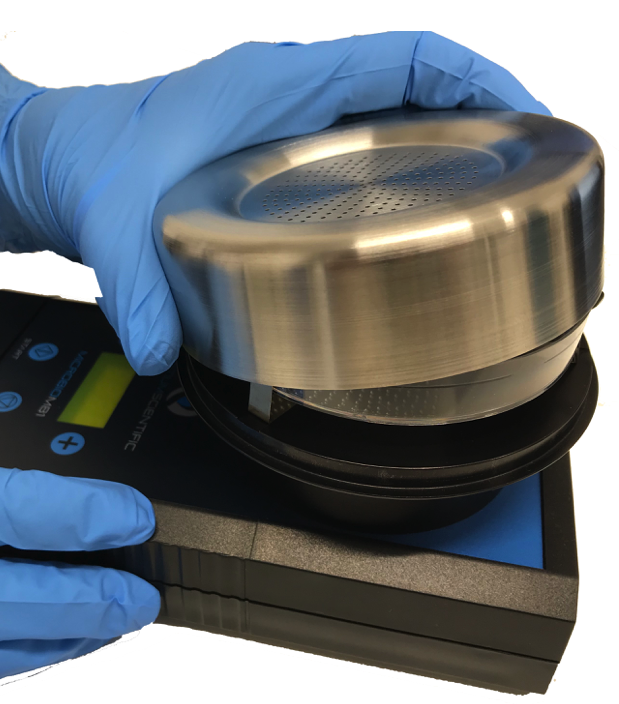
Carefully remove the sampling head cover from the MicroBio MB1, unless it is being carried separately in a sterile container. Only hold the edge of the head - do not touch the perforated or inside surfaces.
With the sampling head removed, insert a petri dish / contact plate inside the springs, so that its base sits firmly on the three support posts, then re-fit the sampling head.

The standard MicroBio MB1 can use 220 or 400 hole sampling heads. The 220 hole sampling head can be used with both 55mm / 65mm contact plates or 90mm petri dishes, but only the centre 55mm of the petri dish will collect samples. Using the 400 x 0.7mm hole sampling head will give nearly full coverage of a 90mm petri dish, improving reliability of counts.
8.3. Switching On and Off
To switch the unit on press any of the four buttons until the display illuminates. If the button is pressed too long (more than 2 seconds), the unit will switch off, preventing inadvertent activation. The MicroBio MB1 will display the last volume setting used.
The MB1 switches-off automatically after 30 seconds, but pressing and holding the STOP button will switch off the MB1 quickly.
When the battery needs re-charging the display will show bat lo on the display. If the battery level is too low, the MicroBio MB1 will not allow samples to be taken, display bat flat on the display and switch off.
Holding down the stop button will get the display to show a battery level percentage.
8.4. Setting Sample Volume

Use the ⊕ or ⊖ buttons to select the volume of air to be sampled.
Volumes can be set as follows:
10 to 200 litres in 10 litre steps
200 to 500 litres in 50 litre steps
500 to 1000 litres in 100 litre steps
1000 to 2000 litres in 250 litre steps
8.5. Starting / Stopping Samples
Press the START button to start the sample process. When running the display will countdown showing how many litres remain to be sampled. The MicroBio MB1 will stop sampling when the required volume of air has been sampled.
Press and hold the STOP button at any time to stop sampling.
When the sample is complete the display will show flashing dashes and an alert tone will sound.
If the battery level is too low the sampler will not start and four dots will show at the bottom of the display.
8.6. Delayed Start
Press and hold START to enable the delayed start feature. This will allow up to 99 minutes of delay, in 1 minute steps, before sampling starts.
Use the ⊕ or ⊖ keys to set the amount of delay required in minutes.
When ready, press START to initiate the countdown. The display will then countdown the time before sampling will start. At any time pressing STOP will cancel the delayed sample.
8.7. Temperature and Humidity
It is useful to take a note of these values at the time of each sample. Temperature and humidity are important factors in the likely concentration and viability of airborne micro-organisms. For example, some bacteria survival rates are 35 to 65 times higher at 80%RH compared to 40%RH.
8.8. Dust Cover (optional)
This accessory protects the media support plate and blower inlet of MicroBio MB1 from contamination during transport and setup. It is manufactured from autoclave-safe 316 grade stainless steel.

9. Determining Results
Once the sample has been taken, the contact plate or petri dish should be removed and sealed with its protective lid. A note should be made on the lid regarding time, location and volume sampled.
The plate should then be incubated for a period of time and temperature dependant on the requirements of the media. Once incubated, the colony growths are then counted, either manually or using an automatic colony counter. Due to the statistical nature of the sampling method and the chance more than one colony impinged at one point on the dish, a count correction needs to be performed.
The tables in Appendix A and Appendix B give the corresponding corrected value for the 220 hole and 400 hole sampling heads respectively.
Alternatively, the following equation can be used to determine a corrected count.
Where nh is the number of holes on the sampling head, nf is the number of counted colonies and nc is the corrected count.
If the counted colonies, (nf) exceeds the number of sampling head holes (nh), then the equation will fail and the results cannot be trusted. If this is the case, then the sample dish can be considered as overloaded with micro-organisms and the user should consider lower sampling volumes.
The colony concentration is the corrected count per volume of air sampled. The results are normally expressed in colony forming units per cubic metre.
To convert the corrected count to CFU/m3 use the equation:
Where nc is the corrected number of colonies counted and Vs is the sampled volume in litres.
An online count correction tool and spreadsheets to automate correction and collation of results are available for free download from:
10. Change Plate / Dish Type
The MicroBio MB1 with a 220 hole sampling head can accommodate both 55mm / 65mm contact plates or 90mm petri dishes. The MicroBio MB1 is factory fitted with the petri dish springs (part number A-00070), but these can be removed and replaced with the supplied contact plate springs (part number A-00068).
The springs for both types have slots to allow a degree of adjustment to suit dish / plate manufacturer variations.
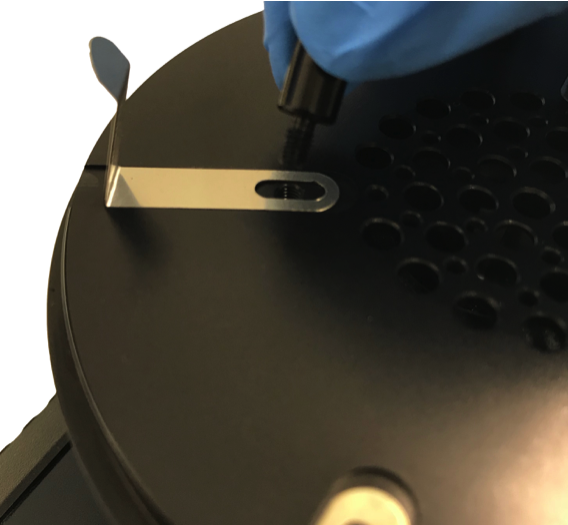
To change the spring type unscrew the three support posts using the supplied 2mm hex-key and remove the springs. Then fit the other alternate springs into the guide slots and screw the support posts back into place. All springs have slots to allow a degree of adjustment to accommodate varying dish sizes.
11. Cleaning
The sampling head should be sterilised preferably by autoclave, but in the field disinfect with suitable sterilising wipes, such as IPA wipes. Wipes may also be used for general cleaning of the sampler body.
Caution: Do not use caustic based cleaning methods.
In areas of low bioaerosol levels, observe a disinfection regime between each sample. Always sample from areas of lower contamination towards those of higher contamination. Wash exposed skin and clothing before sampling and avoid drinking, eating and smoking in the test area or around the equipment. All equipment should be handled aseptically.
The MicroBio MB1 is suitable for use with Hydrogen Peroxide Vapour (H2O2 vapour) bio-decontamination. As the MicroBio MB1 has a fan within the sampling head, it is recommended for optimum decontamination the MicroBio MB1 is set to sample at maximum volume, allowing H2O2 vapour to be drawn through the head, fan and exhaust. Tests have been carried out by Bioquell (UK) Ltd on a range of sensitive electronic equipment to determine effects of such processes. The conditions of these tests were:
Gas Concentration |
1000 ppm |
Conditioning time |
20 minutes |
Gassing time |
30 minutes |
Aeration time |
150 minutes |
Vapour sterilisation above the test conditions levels and times will invalidate the warranty.
Throughout the tests, Bioquell demonstrated that bio-decontamination with H2O2 vapour does not appear to be detrimental, affect operational aspects or aesthetics of sensitive electronic equipment. For further information, please refer to document BDS-3-MATCOMP-V3.2, available from Bioquell (UK) Ltd upon request.
12. Validation
Some industries require sampling equipment to be validated before being used. For the standard MicroBio MB1 with 100 lpm flow rate this can be achieved using the MicroBio Validation Kit, part number A-00058 available from Cantium Scientific Limited or your local distributor.
For the MicroBio MB1 HiFlow model the Qualisair™ qCR kit, part number A-00422, will be required.
13. Calibration
It is recommended the MicroBio MB1 is regularly calibrated in accordance with specific industry best practice. Typically, this will be on the anniversary of the instrument entering service.
The only calibration adjustment on the instrument is the flow rate. Calibration is normally performed with compensation at 20ºC with an air pressure of 1013 mbar.
For calibration services please contact Cantium Scientific Limited or your local distributor. For in-house calibration, we recommend the use of the Qualisair™ qCR kit, part number A-00422 manufactured by Cantium Scientific Limited and available from your local distributor.
14. Troubleshooting
The MicroBio MB1 will give many years of trouble-free service with minimal routine maintenance. However, below are some common questions and answers relating to the use of the MicroBio MB1 Bioaerosol Sampler.
Q. The unit will not switch on.
A. Check the batteries are inserted correctly and fully charged. If this does not resolve the situation, one of the rechargeable cells may be exhausted. Try using standard alkaline cells, otherwise, please contact technical support.
Q. The sampler cuts out and switches off during sampling.
A. This is due to exhausted or low quality batteries. Replace batteries or recharge. Low quality cells may present a high terminal voltage, but as soon as a load is applied, this will drop drastically under load below a point where the sampler will not function properly.
Q. The sampling head fits loosely.
A. There is a retaining spring inside the sampling head area that keeps the head tight. This may, with time and use, have moved. Loosen the screw using the 2mm hex-key supplied with the MB1 kit, slide the spring to the edge of the sampling head support plate and re-tighten. Try this until a secure fit is obtained.
Q. The contact plate or petri dish fits loosely.
A. With time the springs that hold them in place may have loosened. Using the 2mm hex-key supplied, unscrew the support posts, move the springs and re-tighten until a secure hold of the plate / dish is obtained. There is variation in the outside diameter of plates from one manufacturer to another. The MicroBio MB1 holding springs can be adjusted to suit.
If the problem is not covered above, please contact your local distributor for technical support. Otherwise, contact Cantium Scientific Limited via our technical support portal.
14.1. Error Messages
Some faults during operation of the sampler will cause an error message to be displayed on the screen in the form of Err. followed by a number.
Error code |
Fault |
Resolution |
0 |
Memory CRC |
If a memory data error is found, the sampler will correct itself by performing a factory reset. This will only lose the last used volume and delayed start setting. |
1 |
Blower start error |
The sample could not start. Retry starting again after removing battery. If this happens a second time then contact Cantium technical support. |
2 |
Blower stop error |
Under this situation the sample will stop, but the controlled dropping of power to the blower motor failed. If this happens a second time then contact Cantium technical support. |
3 |
Calibration error |
This will be detected at power up, sometimes if the memory has a CRC error, or other data corruption has occurred. The sampler will then revert to a back-up of the calibration setting for the sampler. If this happens repeatedly then contact Cantium technical support. |
4 |
Blower drive initialise fail |
Remove battery then try again. If this happens a second time then contact Cantium technical support. |
5 |
Blower drive voltage fail |
Remove battery then try again. If this happens a second time then contact Cantium technical support. |
14.2. Technical Support
Technical support is available at our technical support portal.
This site provides a search facility for issues, with many support articles and a facility to post questions. Alternatively, contact your distributor.
15. Appendix A - 220 Hole Count Correction Table
Note: For counts greater than those shown in the table below, please refer to ‘Determining Results’ section or visit https://www.cantiumscientific.com/support/count-correction/
Count |
Corrected |
Count |
Corrected |
Count |
Corrected |
Count |
Corrected |
1 |
1 |
41 |
46 |
81 |
101 |
121 |
175 |
2 |
2 |
42 |
47 |
82 |
102 |
122 |
177 |
3 |
3 |
43 |
48 |
83 |
104 |
123 |
179 |
4 |
4 |
44 |
49 |
84 |
106 |
124 |
182 |
5 |
5 |
45 |
50 |
85 |
107 |
125 |
184 |
6 |
6 |
46 |
52 |
86 |
109 |
126 |
186 |
7 |
7 |
47 |
53 |
87 |
110 |
127 |
188 |
8 |
8 |
48 |
54 |
88 |
112 |
128 |
191 |
9 |
9 |
49 |
56 |
89 |
114 |
129 |
193 |
10 |
10 |
50 |
57 |
90 |
115 |
130 |
196 |
11 |
11 |
51 |
58 |
91 |
117 |
131 |
198 |
12 |
12 |
52 |
59 |
92 |
119 |
132 |
201 |
13 |
14 |
53 |
61 |
93 |
120 |
133 |
203 |
14 |
15 |
54 |
62 |
94 |
122 |
134 |
206 |
15 |
16 |
55 |
63 |
95 |
124 |
135 |
208 |
16 |
17 |
56 |
65 |
96 |
126 |
136 |
211 |
17 |
18 |
57 |
66 |
97 |
127 |
137 |
213 |
18 |
19 |
58 |
67 |
98 |
129 |
138 |
216 |
19 |
20 |
59 |
69 |
99 |
131 |
139 |
219 |
20 |
21 |
60 |
70 |
100 |
133 |
140 |
222 |
21 |
22 |
61 |
71 |
101 |
135 |
141 |
224 |
22 |
23 |
62 |
73 |
102 |
136 |
142 |
227 |
23 |
24 |
63 |
74 |
103 |
138 |
143 |
230 |
24 |
26 |
64 |
76 |
104 |
140 |
144 |
233 |
25 |
27 |
65 |
77 |
105 |
142 |
145 |
236 |
26 |
28 |
66 |
78 |
106 |
144 |
146 |
239 |
27 |
29 |
67 |
80 |
107 |
146 |
147 |
242 |
28 |
30 |
68 |
81 |
108 |
148 |
148 |
245 |
29 |
31 |
69 |
83 |
109 |
150 |
149 |
248 |
30 |
32 |
70 |
84 |
110 |
152 |
150 |
251 |
31 |
34 |
71 |
86 |
111 |
154 |
151 |
254 |
32 |
35 |
72 |
87 |
112 |
156 |
152 |
257 |
33 |
36 |
73 |
89 |
113 |
158 |
153 |
261 |
34 |
37 |
74 |
90 |
114 |
160 |
154 |
264 |
35 |
38 |
75 |
92 |
115 |
162 |
155 |
267 |
36 |
39 |
76 |
93 |
116 |
164 |
156 |
271 |
37 |
41 |
77 |
95 |
117 |
166 |
157 |
274 |
38 |
42 |
78 |
96 |
118 |
168 |
158 |
278 |
39 |
43 |
79 |
98 |
119 |
170 |
159 |
282 |
40 |
44 |
80 |
99 |
120 |
173 |
160 |
285 |
16. Appendix B - 400 Hole Count Correction Table
Note: For counts greater than those shown in the table below, please refer to ‘Determining Results’ section or visit https://www.cantiumscientific.com/support/count-correction/
Count |
Corrected |
Count |
Corrected |
Count |
Corrected |
Count |
Corrected |
1 |
1 |
41 |
44 |
81 |
91 |
121 |
144 |
2 |
2 |
42 |
45 |
82 |
92 |
122 |
145 |
3 |
3 |
43 |
46 |
83 |
93 |
123 |
147 |
4 |
4 |
44 |
47 |
84 |
95 |
124 |
148 |
5 |
5 |
45 |
48 |
85 |
96 |
125 |
150 |
6 |
6 |
46 |
49 |
86 |
97 |
126 |
151 |
7 |
7 |
47 |
50 |
87 |
98 |
127 |
153 |
8 |
8 |
48 |
51 |
88 |
100 |
128 |
154 |
9 |
9 |
49 |
53 |
89 |
101 |
129 |
156 |
10 |
10 |
50 |
54 |
90 |
102 |
130 |
157 |
11 |
11 |
51 |
55 |
91 |
103 |
131 |
159 |
12 |
12 |
52 |
56 |
92 |
105 |
132 |
160 |
13 |
13 |
53 |
57 |
93 |
106 |
133 |
161 |
14 |
14 |
54 |
58 |
94 |
107 |
134 |
163 |
15 |
15 |
55 |
59 |
95 |
109 |
135 |
164 |
16 |
16 |
56 |
61 |
96 |
110 |
136 |
166 |
17 |
18 |
57 |
62 |
97 |
111 |
137 |
167 |
18 |
19 |
58 |
63 |
98 |
113 |
138 |
169 |
19 |
20 |
59 |
64 |
99 |
114 |
139 |
170 |
20 |
21 |
60 |
65 |
100 |
115 |
140 |
172 |
21 |
22 |
61 |
66 |
101 |
117 |
141 |
174 |
22 |
23 |
62 |
68 |
102 |
118 |
142 |
175 |
23 |
24 |
63 |
69 |
103 |
119 |
143 |
177 |
24 |
25 |
64 |
70 |
104 |
121 |
144 |
178 |
25 |
26 |
65 |
71 |
105 |
122 |
145 |
180 |
26 |
27 |
66 |
72 |
106 |
123 |
146 |
181 |
27 |
28 |
67 |
74 |
107 |
125 |
147 |
183 |
28 |
29 |
68 |
75 |
108 |
126 |
148 |
184 |
29 |
30 |
69 |
76 |
109 |
127 |
149 |
186 |
30 |
31 |
70 |
77 |
110 |
129 |
150 |
188 |
31 |
33 |
71 |
78 |
111 |
130 |
151 |
189 |
32 |
34 |
72 |
80 |
112 |
131 |
152 |
191 |
33 |
35 |
73 |
81 |
113 |
133 |
153 |
192 |
34 |
36 |
74 |
82 |
114 |
134 |
154 |
194 |
35 |
37 |
75 |
83 |
115 |
136 |
155 |
196 |
36 |
38 |
76 |
85 |
116 |
137 |
156 |
197 |
37 |
39 |
77 |
86 |
117 |
138 |
157 |
199 |
38 |
40 |
78 |
87 |
118 |
140 |
158 |
200 |
39 |
41 |
79 |
88 |
119 |
141 |
159 |
202 |
40 |
42 |
80 |
90 |
120 |
143 |
160 |
204 |
17. Appendix C - Culture Media Types
Micro-organism |
Agar Culture Medium |
Incubation Temperature |
Bacteria: |
||
Human Flora |
Blood Agar |
35 - 37°C |
Possible Pathogens |
Heart Infusion Agar |
35 - 37°C |
Environmental saprophytic |
SCDA or R2A |
25 - 30°C |
Thermophylic |
EMB or Endo Agar |
35 - 37°C |
Fungi: |
||
Environmental saprophytes |
Malt Extract Agar(MEA) |
Room Temp |
|
Sabouraud Dextrose |
Room Temp |
|
Rose Bengal Agar (RBA) (with streptomycin), Inhibitory Mould Agar |
20 - 25°C |
Xerophylic |
Malt Extract Agar with added NaCl, sucrose or dichloran- glycerol |
20 - 25°C |
18. Appendix D - Replacement Parts
Description |
Part Number |
55mm contact plate holding springs |
A-00068 |
90mm petri dish holding springs |
A-00070 |
Head retaining spring |
A-00071 |
2mm hex-key |
A-00206 |
Dish support post |
A-00499 |
Battery charger |
A-00688 |
NiMH rechargeable cell pack |
A-00441 |
220 x 1.0mm stainless steel sampling head |
A-00021 |
400 x 0.7mm aluminium sampling head |
A-00020 |
400 x 0.7mm stainless steel sampling head |
A-00670 |
400 x 1.0mm stainless steel sampling head(HiFlow) |
A-00183 |
MicroBio Dust Cover |
A-00771 |
Padded carry case |
A-00060 |