Crystallization Temperature
The crystallization temperature of a brine is the temperature at which a solid phase begins to form, resulting in a mixture of solid particles and solution. These solids may be salt crystals or water crystals (ice).
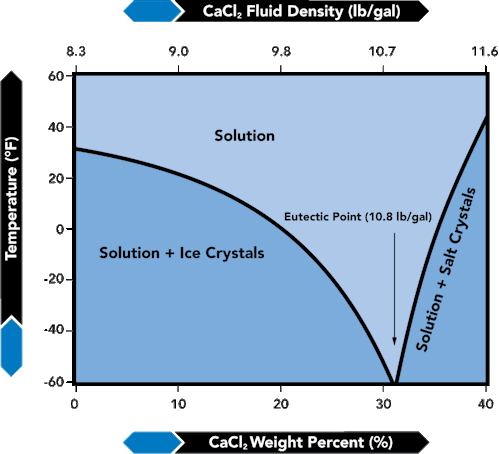
Figure 18 shows a typical crystallization temperature curve for a brine. The system plotted in Figure 18 is CaCl2 - H2O. Note that the left side of the curve slopes downward with increasing CaCl{~}2 concentration levels. This side of the curve is labeled Solution + Ice Crystals. It represents the freezing point of the brine, where ice crystals would begin to form. The right side of the curve is labeled Solution + Salt Crystals. It represents the phase boundary of the brine, below which salt crystals begin to form. The minimum point where the two curves intersect is known as the eutectic point. It is the point at which the minimum crystallization temperature can be realized. In essence, it is the lowest temperature at which a solid free solution (brine) can exist.
FIGURE 18. Crystallization Temperature - Aqueous Calcium Chloride
Virtually all single salt brines have similar phase diagrams. In oilfield applications, one is frequently operating on the right side of the eutectic point. Because of this situation, it is the crystallization temperature that should be the determining factor when selecting a clear brine fluid for a completion/workover application.
FIGURE 19. Typical Crystallization Curve
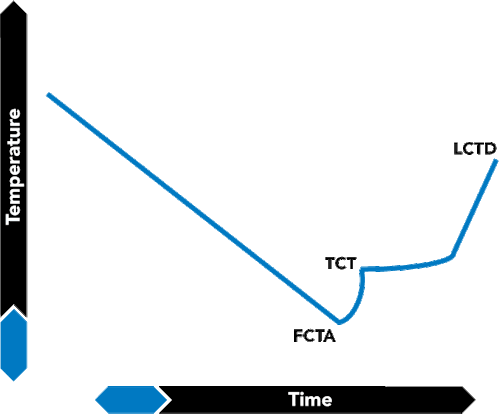
The crystallization point is determined by cooling a brine until salt crystals form, and recording temperatures at various times during the process. Figure 19 shows a typical cooling curve for a brine. Note the three points along the curve. First Crystal to Appear (FCTA) is the point at which salt crystals first form. The formation of salt crystals generates a small amount of heat, which causes a slight rise in the solution's temperature. This higher temperature corresponds to the true crystallization temperature (TCT) of the brine.
Once crystals have formed, the brine can then be heated until all the crystals are redissolved. The point on the curve which corresponds to the temperature at which the salt goes back into solution is labeled Last Crystal to Dissolve (LCTD). As a general rule, the TCT is the most commonly reported crystallization point. In practice, the TCT is extremely valuable since it is a strong reflection of the composition of the brine. It is, in fact, the most reliable and reproducible measure of the safe working limits of heavy brines. The measurement of TCT is governed by an API protocol.
In the case of multisalt brines, the least soluble component will crystallize at the TCT (Table 50). Thus, if a heavy brine is contaminated with minor amounts of NaCl or KCl from formation brine or seawater, the TCT may be shifted to a much higher temperature. This is due to the limited solubility of NaCl and KCl in heavy brines. Although the brine at the altered TCT may appear cloudy, it can be cooled to the original TCT with no further crystallization occurring.
TABLE 50. TETRA Additive RQ Information
Least Soluble Component in Multiple Salt Brines |
|
| Multiple Salt Brines | Least Soluble Component |
| NaCl/CaCl2 | NaCl |
| CaCl2/CaBr2 | CaCl2 |
| CaBr2/ZnBr2 | CaBr2 |
| CaCl2/CaBr2/ZnBr2 | CaCl2 |
Formulation and Specification of Crystallization Temperature
Single salt brines have a unique composition and TCT for a given density fluid. Multisalt brines can be blended to a specific density several different ways, giving rise to a range of TCT formulations. During formulation and the specification of the crystallization temperature of a brine, a TCT should be chosen that is below the minimum expected average ambient temperature. In the case of deepwater applications, the mudline temperature will almost always be the minimum temperature for the entire circulating system. The use of FCTA or LCTD for fluid specification is discouraged. If FCTA is used as the specification, the TCT would be higher than the ambient temperature and would not provide adequate protection against crystallization. Using LCTD would result in a TCT well below average ambient temperature, which would result in the brine being more expensive than necessary.
The TCT of a brine is a critical use parameter for several reasons. First, if crystals form at the surface, the density of the resulting solution is lowered, which can result in pressure control problems. Second, if the TCT is not below ambient temperature, valves and lines can plug quickly, which can cause costly delays in operations. Third, getting the TCT right initially is important because adjusting the TCT at the well site is both costly and time consuming.
Pressurized Crystallization Temperature
TCT is a measure of crystallization temperature under atmospheric pressure. For most applications, the effect of pressure on solubility is slight. However, the effect of pressure can be significant under conditions involving a combination of high pressure and low temperature such as in deepwater applications at seabed or when pressure testing in colder climates. Under these conditions, some brines may crystallize at a temperature higher than the expected TCT, possibly varying by as much as 20°F. Therefore, the pressurized crystallization temperature (PCT) behavior of the brine should be known prior to usage. Unexpected crystallization could have disastrous results. At the mudline, where temperatures are lowest, choke or kill lines can plug and valves can seize, jeopardizing operations. Removal of crystals deposited during pressure testing in the BOP stack or in other locations where circulation is poor can be extremely difficult.
As a general rule, if the composition or density of the brine is represented by a point on the equilibrium curve to the right of the ice-salt eutectic, an increase in pressure will raise the TCT of the brine solution. Recall that salt precipitates at low temperatures for compositions or densities to the right of the ice-salt eutectic point (Figure 18). On the other hand, ice, rather than salt, precipitates for compositions represented by points to the left of the eutectic. A change in volume caused by elevated pressure can lead to a more concentrated solution from which salt will precipitate.
PCT Generalizations
Referring to the crystallization temperature versus weight percent curve in Figure 18:
- The TCT of brines at concentrations greater than the density corresponding to the minimum TCT (to the right of the eutectic point) will be raised by higher pressures in the well, i.e., PCT>TCT.
- The TCT of brines of lower concentrations than the eutectic will be lowered as more pressure is applied to the brine, i.e., PCT<TCT. In Figure 18, these low density brines are shown in the area to the left of the eutectic.
TETRA was the first service company to recognize the potential importance of PCT measurement and to develop the technology enabling such measurement.
|
If you are contemplating a deepwater or subsea completion, learn more about PCT by talking with your TETRA representative and have your fluid tested at TETRA's Technology Center to ensure the formulation of your brine has been adjusted for the effects of PCT. |
