Crystallization Temperature (TCT)
The presence of high concentrations of soluble salts drastically changes the temperature at which, when cooled, crystalline solids begin to form. That temperature is known as the true crystallization temperature. For a more in depth discussion of the relationship between salt concentrations and crystallization temperature and factors influencing the measurement of crystallization temperature, see "Crystallization Temperature" in Chapter 8 of this guide.
Temperature Considerations
Except for low density single salt fluids, most CBFs are near their crystallization temperature or saturation point with respect to one or more of the dissolved salts. Temperature conditions that are likely to be encountered over the length of the fluid column may cause heating or cooling of the brine. Rapid or unanticipated changes in weather conditions may also cause cooling of a fluid as it travels through surface piping and equipment. It is important to anticipate, as closely as possible, the weather conditions that may occur during the entire course of the completion project.
Critical points in the flow path are:
- ocean water surface temperature
- water temperature at the ocean floor (mudline)
- atmospheric conditions—temperature changes in surface tankage and distribution piping due to weather
- filtration equipment
- pill tanks and storage/transfer tanks
If the temperature of a completion fluid is allowed to cool below its stated TCT, solid salts will begin to form. The formation of solids will greatly increase demands placed on pumping equipment due to increased resistance to flow. The solids formed may impede filtration two ways—through a cake buildup in the plate and frame diatomaceous earth (DE) filters and/or by plugging cartridges. Additionally, the formation of solids can result in stuck pipe.
|
The loss of soluble salts, either by settling out or filtration, will drastically reduce the density of the completion fluid. Loss of density could result in a dangerous underbalanced situation. |
It is vital to make a temperature profile for the entire flow system expected for the completion fluids. The lowest temperature likely to be encountered will determine the safe crystallization temperature.
|
To provide an adequate safety margin, the TCT for the fluid should be set 10°F (5.5°C) below the lowest temperature expected to be encountered at any point along the flow path. |
Seasonal Effects and Brine Selection
Crystallization temperature is controlled by the relative proportions of different brine constituents and is affected by environmental factors. A single salt fluid may work during the heat of the summer, whereas at cooler times of the year, a two salt fluid may be required. In other situations, ambient temperatures may dictate the use of a three salt fluid in the winter months, when a two salt fluid might be all that is necessary in the warmer summer months. An 11.6 blend of calcium bromide (CaBr2) and calcium chloride (CaCl2) has a lower TCT than that of a pure calcium chloride (CaCl2) brine of the same density. Adding water can lower TCT, but doing so will result in a loss of density. Along those same lines, zinc bromide (ZnBr2) can be used to reduce the TCT of a two salt calcium chloride-calcium bromide (CaCl2/CaBr2) blend, but the introduction of zinc bromide (ZnBr2) will change the nature of the working brine and will impact the environmental regulations regarding conducting disposal activities and reporting and reacting to spills.
Midrange density fluids, 11.7 lb/gal to 15.1 lb/gal, are typical two salt mixtures of calcium chloride (CaCl2) and calcium bromide (CaBr2). The boundary between two and three salt fluids is influenced by seasonal effects and ocean water temperature at depth. Figure 2 in the Fluid Categories section shows, in a generalized way, the relationship between a brine family and TCT. Values along the vertical axis are density in lb/gal. Colored areas are consistent with those in Figure 3, "TVD-BHP Fluid Density Chart."
| FIGURE 2. Fluid Categories | FIGURE 3. TVD-BHP Fluid Density Chart | |
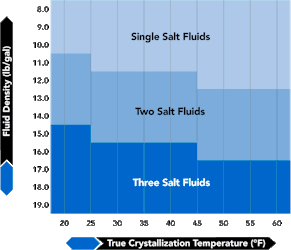 |
 |
|
| Click to display larger image | ||
Pressure Considerations—Pressurized Crystallization Temperature
Deepwater and subsea completions require a greater attention to detail, especially in terms of TCT. At ocean water depths greater than approximately 1,500 feet, an additional adjustment must be made to the fluid formulation. Experience has shown that, at the low temperatures likely to occur in deepwater wells, pressure becomes a factor, and there can be an increase in the measured TCT due to the increase in pressure. At pressures likely to be attained—during the testing of a blowout preventor (BOP) for example—a fluid which functions correctly under normal hydrostatic pressure may begin to crystallize with the increased testing pressure.
TETRA has developed a unique Pressurized Crystallization Temperature (PCT) test designed to measure TCT at various pressures.
|
It is strongly recommended that the PCT be determined for fluids where low temperature and high pressure conditions may coexist. |
If you are contemplating a deepwater completion, ask your TETRA representative to have this unique test performed on your fluid.


